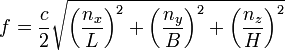Let’s
look at a Planck box that has sides equal to one meter and ask what frequencies
of “light” constitute the standing waves in such a box. With L = B = H = 1
meter in the frequency formula of my previous post, and of course c = the speed of light = 300,000,000
meters per second, which is divided by two, we have
f = 150,000,00(n2x
+ n2y + n2z)1/2 hertz,
= (n2x + n2y
+ n2z)1/2 (1.5 X
108) Hz.
This is meant to be a picture in mathematical form that shows the meaning
of the word mode: put in integers for nx, ny, and nz
and you have chosen a particular mode, which you can see also corresponds to a particular
frequency. But there are several modes for each frequency. For instance, the
1,1,0 mode has the same frequency as the 1,0,1 and 0,1,1 modes. This is the
lowest frequency mode that can be supported by this box (for the 1,0,0, and
0,1,0 and 0,0,1 modes, wave motion doesn’t happen).
As you can see, this lowest frequency is1.5 X 108Hz.
My bad... That's gotta be multiplied by the square root of two, as you can see in the equation when you put in ones for two of the n's. The square root of two is 1.414. Putting that in gives the famous Manhattan area code, formerly the only one for Manhattan, in megahertz
This is in the VHF (very high frequency) region where FM radio and lower-frequency TV stations broadcast (in fact this is almost exactly the carrier frequency of channel 13). UHF stations broadcast at higher frequencies, and cell phones transmit and receive at microwave frequencies which are even higher in frequency. All these are far below the frequencies of visible light.
As you can see, this lowest frequency is
f = 2.12 X 108 Hz = 212 X 106 Hz = 212 MHz.
This is in the VHF (very high frequency) region where FM radio and lower-frequency TV stations broadcast (in fact this is almost exactly the carrier frequency of channel 13). UHF stations broadcast at higher frequencies, and cell phones transmit and receive at microwave frequencies which are even higher in frequency. All these are far below the frequencies of visible light.
This
equation for frequencies in the one-cubic-meter box (approximately a
one-cubic-yard box, for those of you not familiar with the meter) shows the normal modes of electromagnetic waves that can
exist in the box. What we really want to know is how such a box can produce electromagnetic (EM) waves in the first place, and what higher-frequency waves--even into the visible spectrum--can be produced.
You remember how, right? The box is heated to a certain temperature and a spectrum of EM radiation is produced that is uniquely associated with that temperature: the Planck spectrum. The Planck spectrum is observed when the walls of the box have equal rates of absorption and emission of electromagnetic energy, in other words when thermodynamic equilibrium is established. We’re getting back to thermodynamics now, and will be looking next at how temperature affects the frequencies produced by our cubic meter box.
You remember how, right? The box is heated to a certain temperature and a spectrum of EM radiation is produced that is uniquely associated with that temperature: the Planck spectrum. The Planck spectrum is observed when the walls of the box have equal rates of absorption and emission of electromagnetic energy, in other words when thermodynamic equilibrium is established. We’re getting back to thermodynamics now, and will be looking next at how temperature affects the frequencies produced by our cubic meter box.